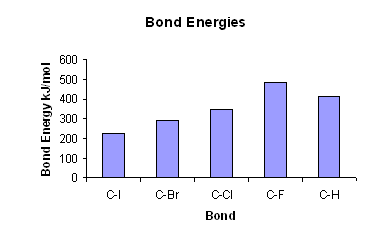
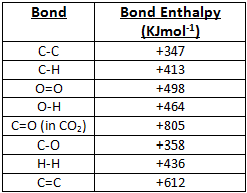
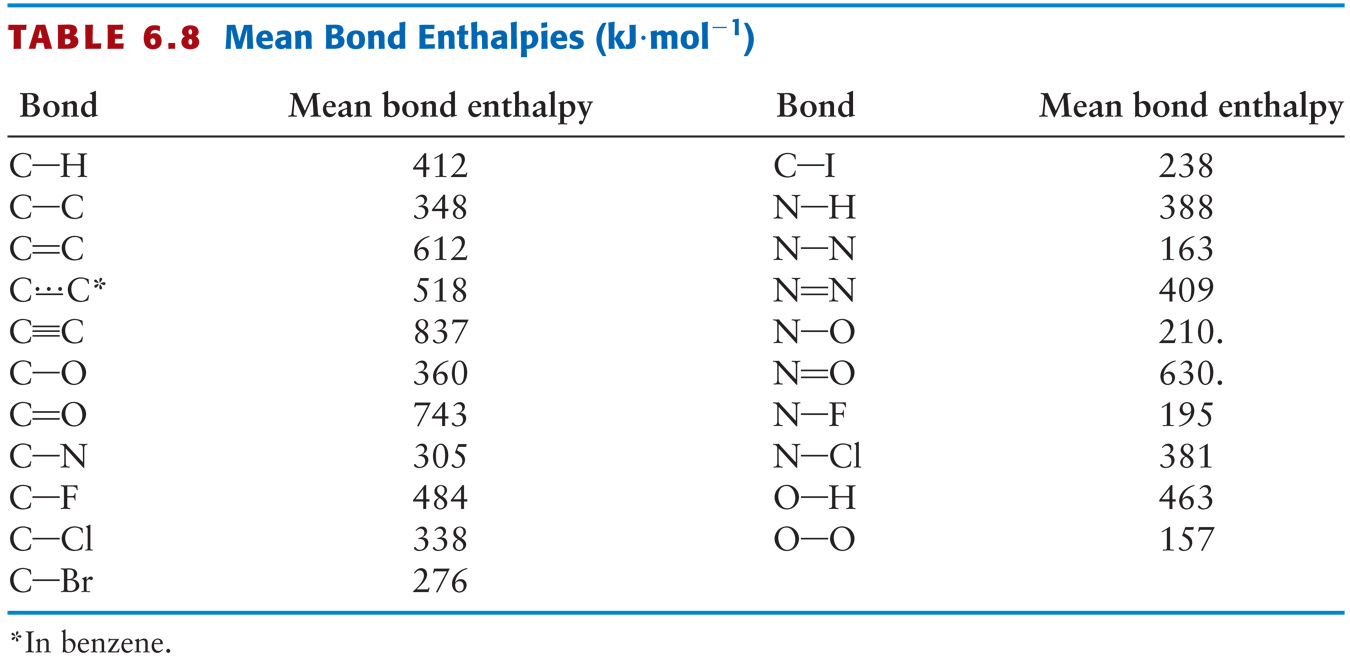
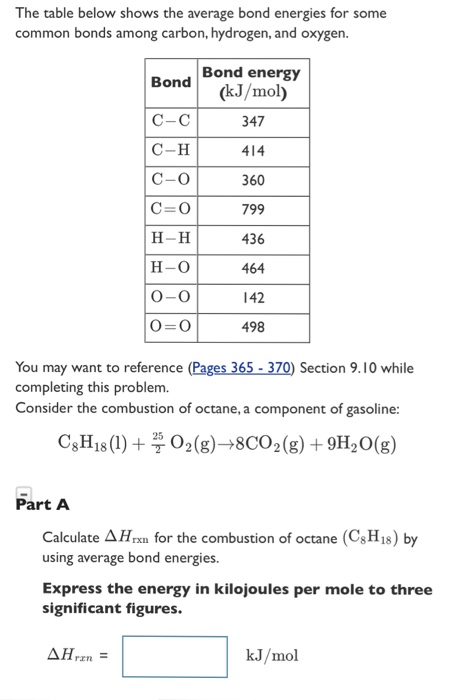
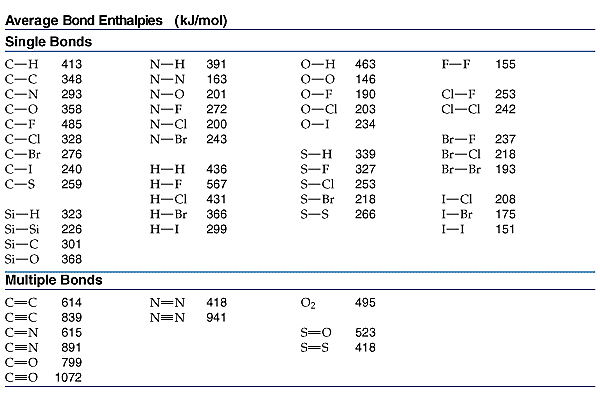
A level Bond Enthalpy (bond dissociation energy) calculations for Enthalpy of Reaction KS5 GCE chemistry revision notes

Chemical reactions are invariably associated with the transfer of energy either in the form of heat or light. In the laboratory, heat changes in physical and chemical processes are measured with an

Chapter 8 General Bonding Concepts. 8.1: I. Types of Chemical Bonds A. Determines behavior/properties of compounds -ex. Carbon can form graphite or diamonds. - ppt download

Bonding energy potential and carbon-carbon bond-length distribution.... | Download Scientific Diagram