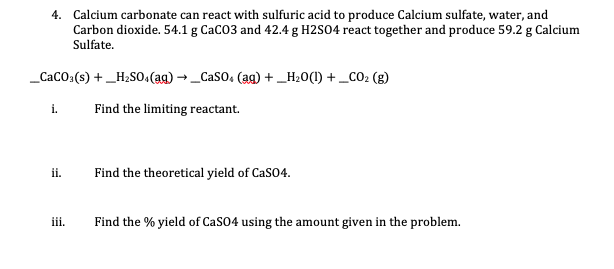
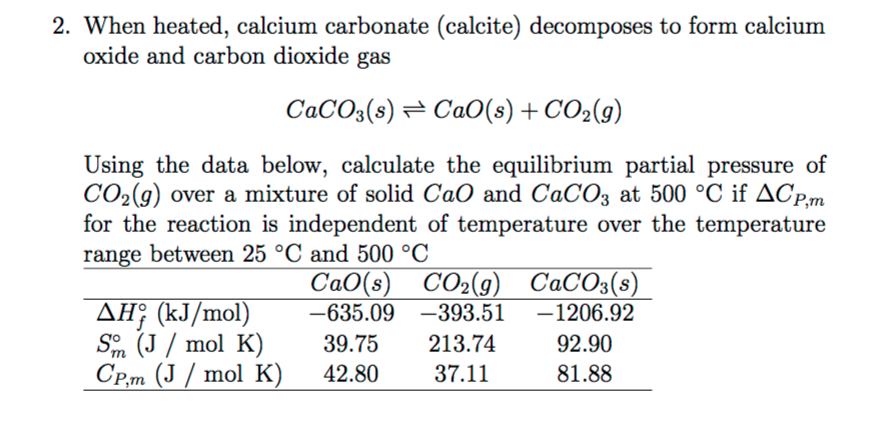
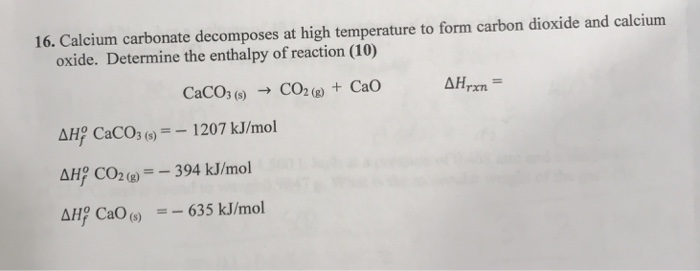
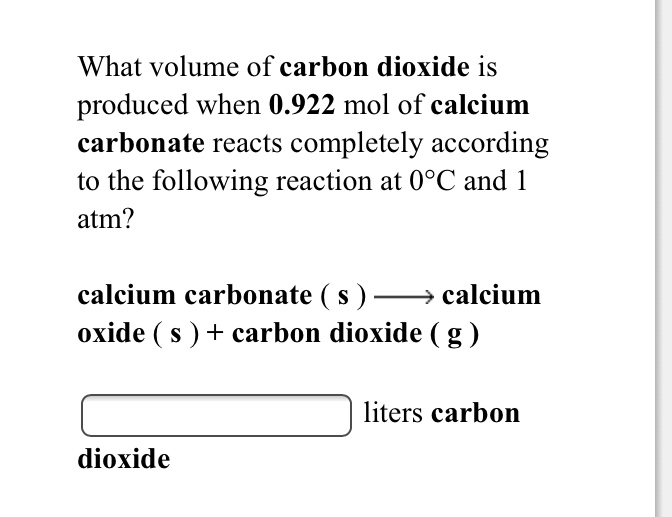
To prepare carbon dioxide gas, 25.0 of calcium carbonate is added to a solution in which 20g hydrogen chloride has been dissolved. The unbalanced reaction is CaCo3 +HCl=CaCl2 +CO2+H2O…determine the limiting reagent? -
SOLVED: If calcium carbonate is heated strongly, carbon dioxide gas is driven off, leaving a residue of calcium oxide. Write the unbalanced chemical equation for this process.
Frontiers | Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism

Write the balance chemical equation for the following reactionsCalcium hydroxide + carbon dioxide → Calcium carbonate + WaterZinc + silver nitrate → Zinc nitrate + SilverAluminium + Copper chloride → Aluminium Chloride + Copper
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate - Energy & Environmental Science (RSC Publishing)

Carbon dioxide conversion into calcium carbonate nanoparticles using membrane gas absorption - ScienceDirect
Write symbolic representation for the following word equations and balance them : (a) Calcium carbonate → Calcium oxide + Carbon dioxide - Sarthaks eConnect | Largest Online Education Community
How much volume of carbon dioxide is produced when 50 g of calcium carbonate is heated completely under standard conditions? - Sarthaks eConnect | Largest Online Education Community
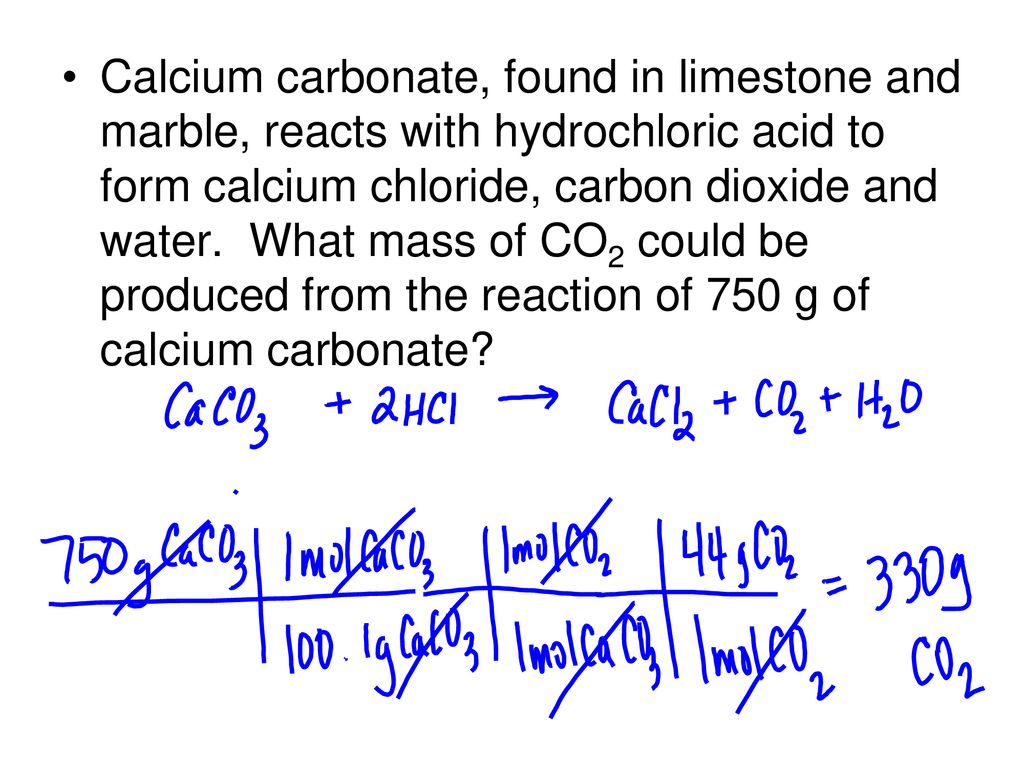
Calcium carbonate, found in limestone and marble, reacts with hydrochloric acid to form calcium chloride, carbon dioxide and water. What mass of CO2 could. - ppt download
CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system - RSC Advances (RSC Publishing)

Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa

Reaction steps involved in the formation of the CaCO3 sol from calcium... | Download Scientific Diagram
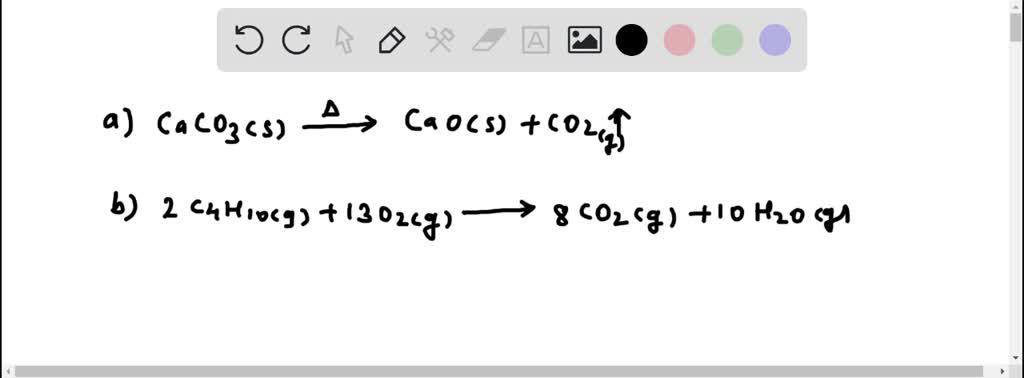
SOLVED:Write a balanced molecular equation describing each of the following chemical reactions. (a) Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. (b) Gaseous butane, C4