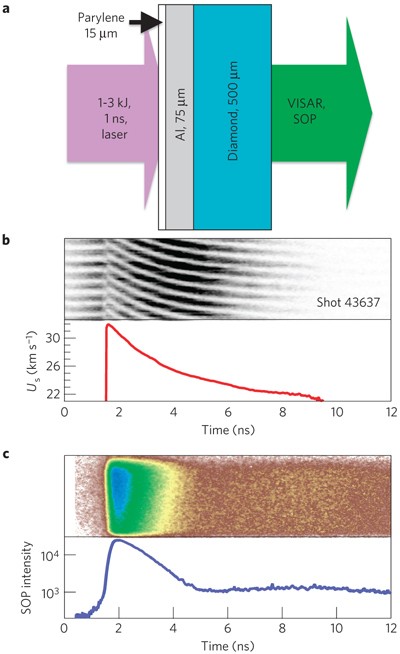
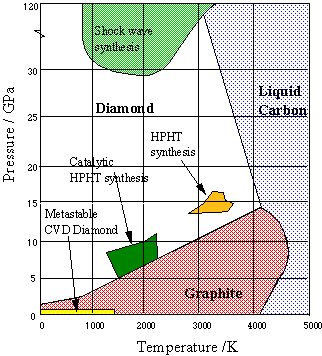
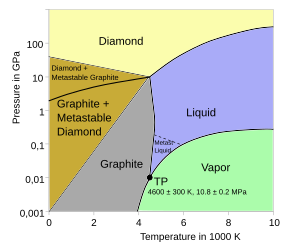
Phase diagram of carbon. M is diamond melting curve; (a)-(g) are shock... | Download Scientific Diagram
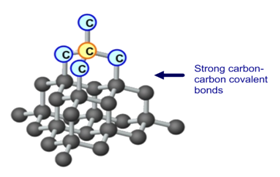
1:49 explain why substances with giant covalent structures are solids with high melting and boiling points - TutorMyself Chemistry
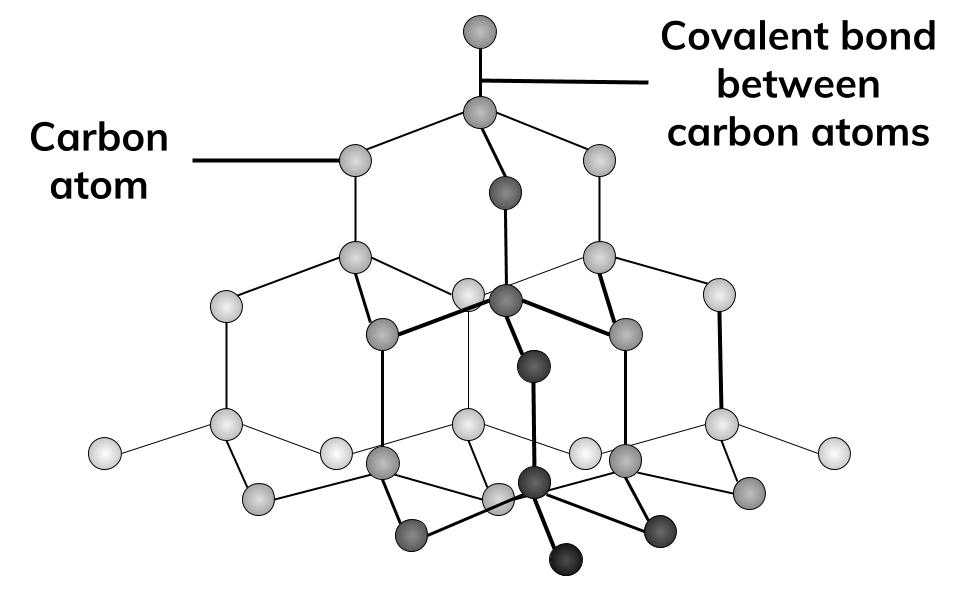
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry

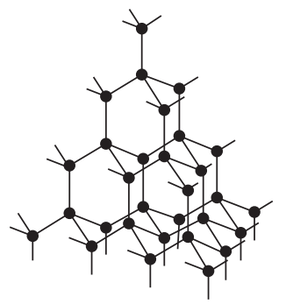
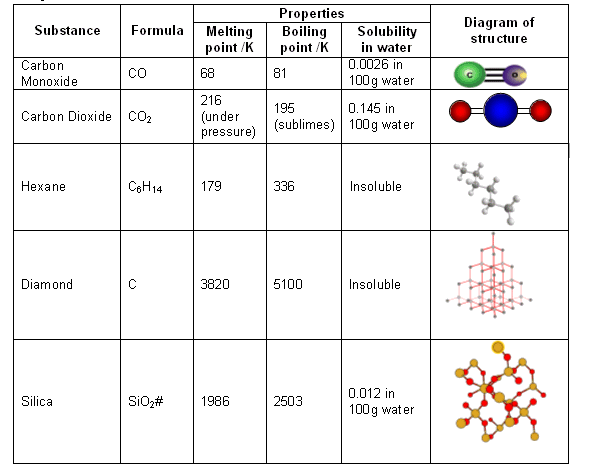
a What is diamond Of what substance is diamond made b Describe the structure of diamond Draw a simpl...