PDF) Promoting psychosocial well-being following stroke: Study protocol for a randomized, controlled trial

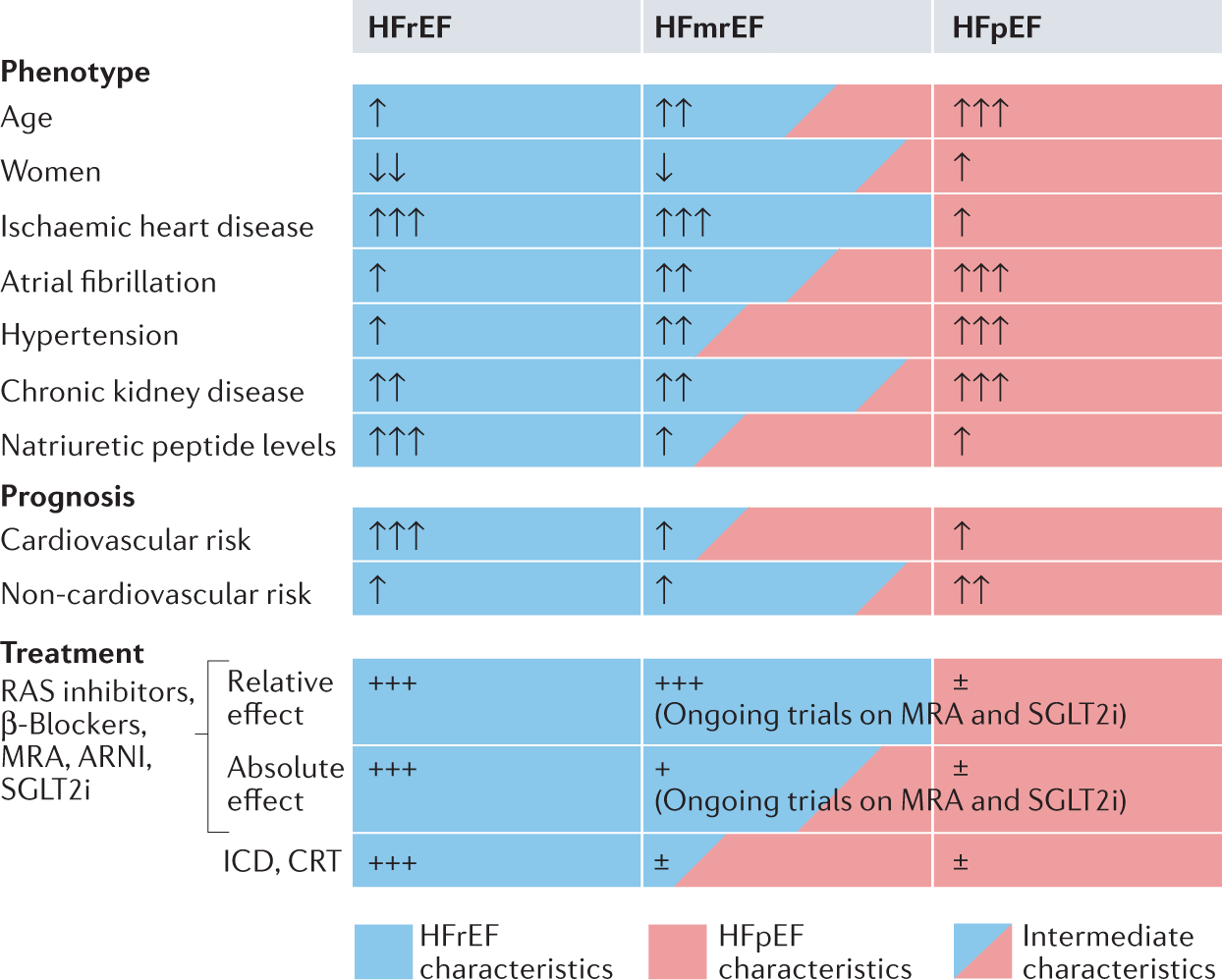
Baseline Characteristics of Patients With HF With Mildly Reduced and Preserved Ejection Fraction: DELIVER Trial | JACC: Heart Failure
![PDF] Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial | Semantic Scholar PDF] Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ff2a649ad2496775a497804af4658a751daade79/3-Figure2-1.png)
PDF] Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial | Semantic Scholar

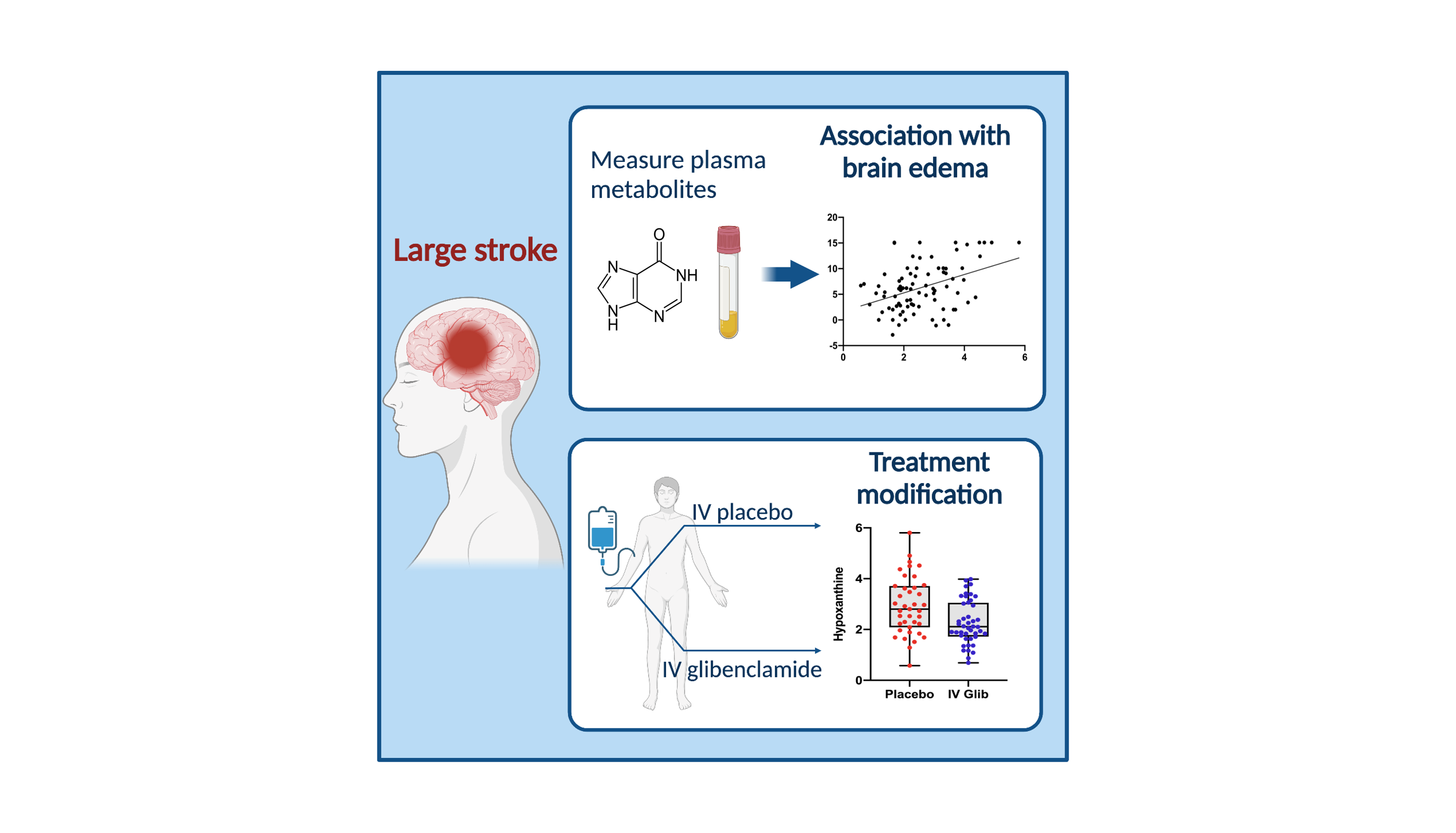
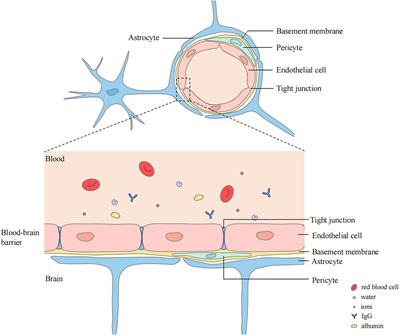
Cerebral Edema in Patients With Large Hemispheric Infarct Undergoing Reperfusion Treatment: A HERMES Meta-Analysis | Stroke

Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial - The Lancet

Phase 3 Study to Evaluate the Efficacy and Safety of Intravenous BIIB093 (Glibenclamide) for Severe Cerebral Edema Following Large Hemispheric Infarction (CHARM) > Clinical Trials > Yale Medicine

Myocardial Infarction in Heart Failure With Preserved Ejection Fraction: Pooled Analysis of 3 Clinical Trials - ScienceDirect

Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial - The Lancet

Promoting psychosocial wellbeing following stroke using narratives and guided self-determination: a feasibility study – topic of research paper in Psychology. Download scholarly article PDF and read for free on CyberLeninka open science

Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial - The Lancet

Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial - The Lancet
Acute stroke trials invite patients to participate within a short time period- including up to 12hrs, 72hrs, 96hrs and 120 hours